Established in 2003, OpSens initially developed sensor technology for use in oil and gas, industrial applications, and by some medical device manufacturers to measure temperature, pressure, and displacement. Their success in these sectors quickly established their reputation as experts in sensor technology. However, the team recognized the broader potential of their unique systems, seeing an opportunity to make a significant medical impact on a larger scale. In 2009, OpSens Medical expanded its focus to the global fractional flow reserve (FFR) market, shifting to cardiology applications. The company honed its expertise to meet the growing need for more sophisticated measurement, documentation, and evaluation tools for cardiologists and their patients.
From the outset, Lumira Ventures forged a strong relationship with the OpSens team. While initially cautious about the early stages of their efforts, we recognized the potential to benefit patients and closely monitored the company’s growth. As OpSens shifted its focus to developing pressure guidewires for cardiac care, we were impressed by the exceptional progress they made. In 2016, we became steadfast supporters, investing in OpSens Medical with the belief that this was a unique opportunity to reinvent coronary disease care and introduce best-in-class solutions that would significantly improve patient outcomes. Coronary artery disease, the leading cause of heart disease and death in North America, is a challenge that OpSens continues to address with its versatile technology, which outperforms traditional standards of care and resolves critical clinical limitations. As the company continues to make exceptional strides, we are proud to be part of introducing the new gold standard in interventional cardiology.
Solution: The OptoWire™, SavvyWire™, and OptoMonitor™

The OptoWire™ is an advanced pressure guidewire powered by Fidela, a second-generation fiber optic sensor. It allows physicians to measure the Fractional Flow Reserve (FFR) and Diastolic Pressure Ratio (dPR) with high precision, helping determine the severity of coronary artery blockages. Its design mimics that of a standard guidewire, with enhanced resistance, flexibility, and sensitivity. This combination of attributes enables physicians to use a single device to obtain multiple measurements across complex structures with confidence in the accuracy of results. Additionally, the wire’s durability supports stenting and balloon angioplasty without the need to replace it, preserving measurement accuracy. With the ability to disconnect and reconnect, physicians can easily adjust to varying anatomies, perform interventions, and confirm improvements following stent implantation.

The SavvyWire™ is the first and only 3-in-1 sensor-guided solution for Transcatheter Aortic-Valve Replacement (TAVR) procedures. Designed to improve TAVR efficiency and enhance long-term patient management, the SavvyWire ensures stable aortic valve delivery and positioning with continuous hemodynamic measurements during the procedure. It also offers reliable ventricular pacing without the need for adjunct devices or venous access.
The OptoMonitor™ system, a paired smart integration tool, enables real-time measurement management directly at the table or through integrated monitoring systems. Physicians can instantly compare pressure measurements from the artery before and after blockage, providing faster, more actionable diagnoses and decisions, ultimately allowing patients to return home sooner and free from symptoms.
Generating Impact Today
Clinical studies have shown the substantial impact of pressure guidewires on both patient outcomes and clinical practice [1]*.
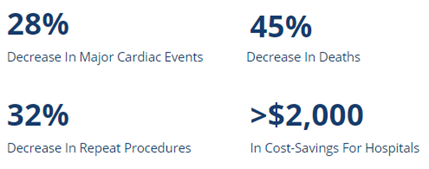
Today, the OptoWire™ is the gold standard of care, having been instrumental in diagnosing and treating coronary artery disease. To date, having benefitted 200,000 + patients with coronary artery blockage in over 30 countries around the world [1]*.
This rapid adoption has led to the installation of more than 2,000 OptoMonitor™ units worldwide, further enhancing clinical success rates in challenging coronary interventions. The OptoWire™ is currently approved for sale in the United States, the European Union, Japan, and Canada, with global sales channels in place.
OpSens’ impact extends beyond coronary artery disease. The company is now leveraging its expertise to expand into structural cardiology with the SavvyWire™. With the global TAVR market projected to exceed 400,000 procedures by 2027, the SavvyWire™ is poised to make a significant impact on patients worldwide. Approved by Health Canada and the U.S. FDA, the SavvyWire™ has already been used in procedures across the U.S. and Europe, with recent expansions to the Pacific coast of New Zealand [1]*.
Learn more about OpSens Medical
Source List:
[1] Source: OpSens Medical, www.opsens.com, 2024
*All statements have been reviewed and validated by Bardy Diagnostics. References and supporting information can be found on the company’s official website.